FDA Cites Investigation, Manufacturing, & Quality Issues in Warning Letter

FDA Warning Letter Summary
In Warning Letter 320-25-22 after an FDA inspection, the FDA cited the following significant deviations from Current Good Manufacturing Practices (CGMP):
- Failure to investigate all critical deviations.
- Failure to demonstrate that your manufacturing process can reproducibly manufacture an API meeting its predetermined quality attributes.
- Failure to have equipment of the appropriate design and suitability for their intended use for the manufacture of APIs.
- Failure of your quality unit to exercise its responsibility to ensure the API manufactured at your facility are in compliance with CGMP.
- Failure to ensure all production deviations are reported and evaluated, and that critical deviations are investigated, and the conclusions are recorded.
What does the FDA want the manufacturer to do? The letter says:
“Correct any deviations promptly. Failure to promptly and adequately address this matter may result in regulatory or legal action without further notice including, without limitation, seizure and injunction. Unresolved deviations may also prevent other Federal agencies from awarding contracts.”
One Suggested Action
All pharmaceutical manufacturers need a superior program to:
- Investigate,
- Find the root causes of,
- And develop effective corrective actions for,
all significant deviations from Current Good Manufacturing Practices.
What do we recommend? TapRooT® Root Cause Analysis.
TapRooT® Root Cause Analysis helps quality professionals:
- Perform complete, accurate investigations,
- Identify the root causes of human performance and equipment failures,
- Develop effective corrective actions to help stop repeat incidents.
Are your quality professionals ready for effective incident investigations, root cause analysis, and corrective actions? With the quality, public relations, and financial risks drug manufacturers face, management should be looking for the best investigation, root cause analysis, and corrective action processes. Perhaps management should consider sending people to our 5-Day TapRooT® Advanced Root Cause Analysis Team Leader Courses. See our upcoming public TapRooT® Courses HERE. Or CONTACT US for a quote for a course at your site.

Going Beyond Great Root Cause Analysis
If you want your CAPA/improvement program to shine, I suggest attending the Improvement Program Best Practice Track at the 2025 Global TapRooT® Summit, which will be held September 29 – October 3 in Knoxville, Tennessee. What topics are in the Improvement Program Best Practices Track?

For details about each topic, CLICK on the image above.
Another Track to consider is the High-Reliability Organization Best Practices Track at the Summit. After all, shouldn’t pharmaceutical manufacturers be high-reliability organizations?
What is in the High-Reliability Organization Best Practices Track?

For details about eash session in the track, CLICK on the image above.
Of course, there’s more to the 2025 Global TapRooT® Summit than the best practice tracks. There are five Keynote Speakers.

For more information about the Summit, watch thhis video…
On September 29-30, there are 11 pre-Summit Courses to consider.
By attending a course or bringing a team to the Summit, you can save. See potential discounts up to $900 OFF per attendee below.
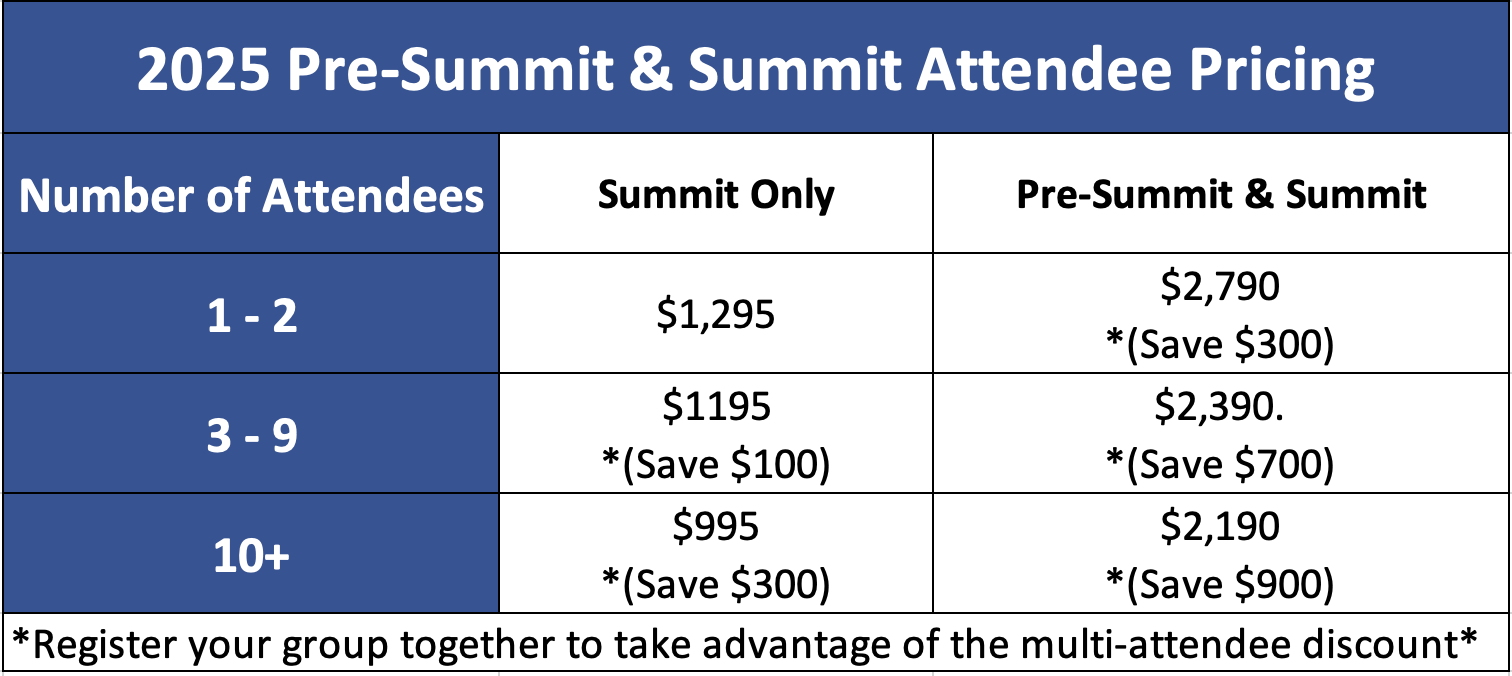
To register for the Summit, CLICK HERE.




